Polyoxometalates (POMs)
are large inorganic anions formed via condensation-addition reactions
of simple oxoanions of early transition elements such as V, Mo and W.
e.g.
7 MoO42- + 8 H+ =
[Mo7O24]6- + 4 H2O
an isopolymolybdate
Generally, such polyanions have symmetric close-packed
structures, they have high thermal and hydrolytic stabilities (over a characteristic
range of pH) and are formed rapidly in solution by "self-assembly" processes.
| We show four examples of how the properties of POMs may be exploitable for the treatment of radioactive waste involving lanthanides, actinides, and technetium. |
1.
Selective separation and binding of Ln3+ and An4+
The tungstophosphate anion
[Na(H2O)P5W30O110]14-
is a doughnut-shaped anion of C5v symmetry, with a central cylindrical
cavity which encloses a sodium cation and axially coordinated water molecule,
see Figure 1.
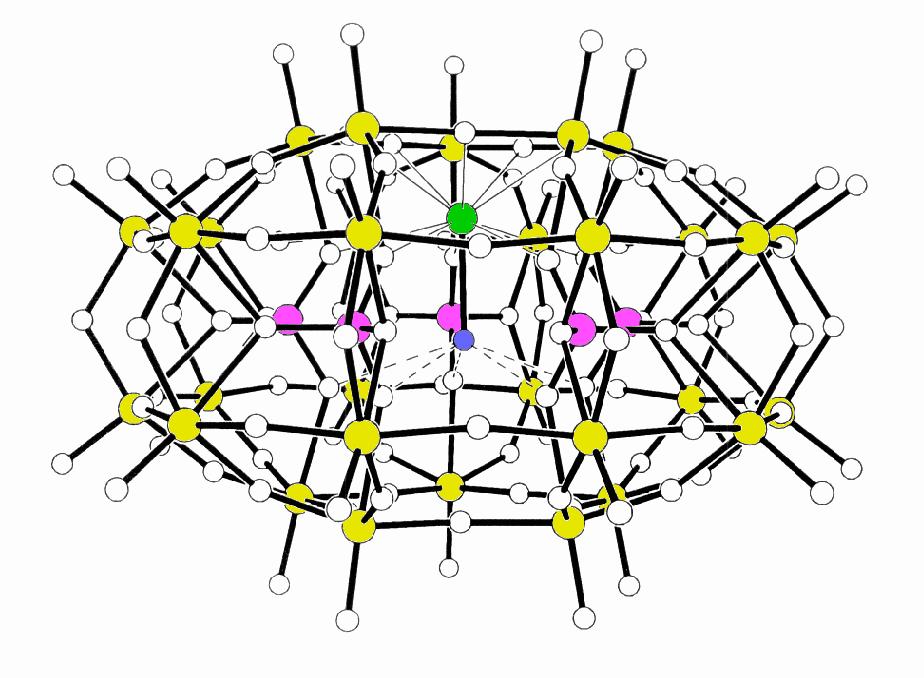
Figure 1(a)
Figure 1(b)
Figure 1(a). Polyhedral Representation
of [Na(H2O)P5W30O110]14-
viewed approximately down the molecular C5 axis
Figure 1(b). Ball-and-stick view of
[M(H2O)P5W30O110]n-
perpendicular to the C5 axis showing position of the internal
cation (green) and its associated water molecule
(blue). Pink circles are the phosphorus atoms
The anion is stable in aqueous solution between pH
< 0 (6 M HCl) and pH 10.
Under vigorous hydrothermal conditions (e.g. 170oC,
24h) the central cation can be replaced with cations of similar size
i.e. Ca2+, Y3+ and most Ln3+, Th4+
and U4+.
Eu3+ + [Na(H2O)P5W30O110]14-
--------> [Eu(H2O)P5W30O110]12-
+ Na+
Cations that are significantly smaller or larger
than Na+ (e.g. Al3+, transition metals) are excluded.
The following results (Figure 2) show that the replacement reactions are kinetically controlled and that the more highly charged cations compete effectively with excess Na+, a major component of high level waste solutions.
Reaction:
M3+ + [Na(H2O)P5W30O110]14-
------->
[M(H2O)P5W30O110]12-
+ Na+ at 170oC
LnIII = La, Ce, Nd, Sm
(30% isolated yield as ammonium salt)
|
|
 |
 |
|
|
4. Complexation of Technetium by Niobates and Niobotungstates
Initial experiments have been carried out using rhenium as a nonradioactive surrogate for technetium. Although this investigation is still in an early stage, encouraging results have been obtained with the hexaniobate anion which stabilizes Re(IV) in highly alkaline solutions (pH 12 or greater). The complexes are believed to be analogous to those of Mn(IV) and Ni(IV) that we had characterized several years ago. Isolation of these and analogous complexes with niobotungstates is in progress, and conversion of these salts to inert mixed oxide materials as possible waste forms is planned. Parallel experiments with the corresponding technetium species will be carried out collaboratively at Oak Ridge National Laboratory.
"Heteropoly
and Isopoly Oxometalates", Pope, M.T. ; Springer-Verlag, New York,
1983. (pp. 1-180).
"Isopolyanions and Heteropolyanions",
Pope, M.T. in Comprehensive Coordination Chemistry, Wilkinson,
G.; Gillard, R.D.; McCleverty, J.A. eds., Volume 3, pp. 1023-1058,
Pergamon Press, 1987.
"Polyoxometalates. From Platonic
Solids to Anti-Retroviral Activity", Pope, M.T.; Müller, A., eds.
Kluwer Academic Publishers, Dordrecht, The Netherlands, 1994, pp 1-411
"Polyoxoanions", Pope, M.T. in Encyclopedia
of Inorganic Chemistry, King R.B. ed., John Wiley &Sons, Chichester,
England, 1994, 3361-71
“Polyoxometalates: Very Large Structures
- Nanoscale Magnets”, Müller, A.; Peters, F.; Pope, M.T.; Gatteschi,
D., Chem.Rev. 1998, 98, 239-271
"A Heteropolyanion with Fivefold Molecular
Symmetry that Contains a Nonlabile Encapsulated Sodium Ion. The Structure
and Chemistry of [NaP15W30O110]14-"
Alizadeh, M.H.; Harmalker, S.P.; Jeannin, Y.; Martin-Frere, J.; Pope, M.T.
J. Am. Chem. Soc. 1985, 107, 2662-2669.
"Rigid Non-Labile Polyoxometalate Cryptates
[ZP5W30O110](15-n)- that Exhibit
Unprecedented Selectivity for Certain Lanthanide and Other Multivalent
Cations" Creaser, I.; Heckel, M.; Neitz, R.J.; Pope M.T., Inorg. Chem.,
1993, 32, 1573-1578.
"The Structures of Europium(III)- and
Uranium(IV) Derivatives of [P5W30O110]15-.
Evidence for Cryptohydration", Dickman, M.H.; Gama, G.J.; Kim, K.-C.; Pope,
M.T., J.Cluster Sci., 1996, 7, 567-583
"Self-Assembly of Supramolecular Polyoxometalates.
The Compact, Water-Soluble Heteropolytungstate Anion [AsIII12CeIII16(H2O)36W148O524]76-,
Wassermann, K.; Dickman, M.H.; Pope, M.T., Angew.Chem., 1997,
109, 1513-1516; Angew.Chem.Internat.Ed.Engl.,1997,
36, 1445-1448
"Formation
of Cerium(III)- and Uranium(IV)-Tungsten Bronzes Through Thermal Degradation
of Polyoxometalates", Wassermann, K.; Pope, M.T.; Salmen, M.; Lunk, H.J.
Abstracts of Papers presented at the Annual Meeting of the German Chemical
Society (Gesellschaft Deutscher Chemiker), Saarbrücken, Germany, 9/23-25/98